Research Article - Diabetes Management (2016) Volume 6, Issue 3
A single-blind, Randomized, Singlecentre Study to Investigate the Characteristics of Different Personal Lancets on Blood Volume and Perceived Pain in Patients with Diabetes Mellitus
- Corresponding Author:
- Gajane Żurawska
Marketing Director, HTL-STREFA
Marketing, Adamowek 7, Ozorkow, 95-035
Poland
Tel: +48607813268
E-mail: katarzyna.mackiewicz@htl-strefa.pl
Abstract
Introduction: Randomised controlled trial is a specific type of scientific experiment, and the gold standard for a clinical trial. RCTs are often used to test the efficacy or effectiveness of various types of medical intervention within a patient population. RCTs may also provide an opportunity to gather useful information about adverse effects. In recent years the performance and accuracy of self-monitoring blood glucose (SMBG) devices have been closely watched by regulatory agencies and medical professionals. Mainly, because every day thousands of patients with diabetes lance their fingers many times in order to perform self-monitoring of their blood glucose values.
Material and methods: Male and female subjects with diabetes type I or II being in good physical and mental health were enrolled in the study. Female subjects were allowed to participate in this study only if they were not pregnant in self reporting. In total 60 diabetic patients were enrolled in the study. The primary objective of this study was to determine and to compare the amount of capillary blood volume collected after a single lancing of the fingertip. In addition, also the perceived pain during the lancing procedure was determined and compared between the lancets.
Results: The average blood volumes obtained with Droplet® personal lancet 33G and Glucoject® personal lancet 33G used in cooperation with Droplet® lancing device, Microlet® 2 lancing device and Glucoject® lancing device were in each case higher than 4 μl except for one puncture. In 50% of pricks the obtained average blood volume was higher than 6 μl. Bleeding time was up to 2 minutes. The study results have also shown that in case of majority of patients the received blood sample volume has been higher than 0.5 μl with a total effectiveness rate of 91.67%. Perceived pain was evaluated as a secondary efficacy variable. The pain perception was measured after each prick. 5 minutes (+/- 1 minute) after pricking the subject noted in his/ her worksheet intensity of the perceived pain.
Conclusions: As demonstrated by the results of clinical evaluations, Droplet® personal lancets 33G type 560 and Glucoject® personal lancets 33G type 560 manufactured by HTL-STREFA S.A. provide sufficient blood sample for personal blood glucose level measurements with minimal pain perception.
Keywords
type 1 diabetes, type 2 diabetes, personal lancet, andomized study
Introduction
Recent guidelines recommend that all patients with type 1 diabetes measure their blood glucose at least 3 to 4 times/day. For other types of diabetes, different recommendations were made, while for individuals with type 2 diabetes and none insulin dependent (NIDD), the proposals for self-monitoring blood glucose (SMBG) are controversial, ranging from frequent testing to no SMBG testing at all.
The importance of tight glycaemic control for avoiding complications of diabetes have been shown in several studies [1,2]. Overall, the medical benefits of close blood monitoring in diabetes patients are widely accepted and supported by the recent literature.
However, frequent blood sampling is inconvenient, costly, relatively painful and the frequent use over several years leads to issues with finger sensitivity.
These factors are major issues why patients are reluctant to perform SMBG frequently and are noncompliant with therapy recommendations. Reducing the number of SMBG measurements very often leads to suboptimal metabolic control (high values of HbA1c) which then is often followed by an increase of diabetes-related complications. Therefore, any reduction of barriers to perform SMBG is highly relevant for patients to help them avoid serious complications of diabetes [3].
Furthermore, both adults and children, use the same size lancets as no lancets suiTable for diabetic children are available.
In other words, reducing lancing pain has the potential to improve patient compliance with respect to their adequate glycaemic control and will definitely improve the quality of life of diabetes patients [4]. In addition, modern glucose meters only require a small amount of blood for an accurate and precise measurement, therefore diabetic patients no longer need to use old lancets with a large size needles or blades.
Material and Methods
▪Study design
The study has been conducted with the highest respect for the individual participants in accordance with the requirements of this Clinical Trial Protocol and also in accordance with the following:
• World Health Organization (WHO) Declaration of Helsinki (sixth revision 2008)
• CH Harmonized Tripartite Guideline for Good Clinical Practice
• Respective local laws and regulations
The study was positively evaluated/reviewed by the Bioethics Committee at the Regional Medical Chamber in Krakow, Poland.
The primary objective of this study was to determine and to compare the amount of capillary blood volume collected after a single lancing of the fingertip. The pain perceived during the lancing procedure was determined and compared between the lancets. The primary endpoint of this study was capillary blood volume produced during each lancing. The pain perceived was regarded as secondary endpoint.
This was a single blind, randomized, singlecentre study in male and female patients with Diabetes mellitus type I or type II. The subjects came to the medical site on the scheduled day for 2-3 hours. After they have signed the informed consent form, they were randomized to one of the ten different finger rotation sequences. Thereafter the finger pricking (three times) was conducted in a standardized fashion. Shortly after the last pricking the CRF was checked for completeness, dated and signed by the investigator or designee and the subject was released. After the completed screening and randomization procedure the lancing procedure was performed.
Finger pricking was undertaken in a standardized fashion and performed by the same investigator or designee. Before the start of the lancing procedure the volunteers were washing their hands with soap under warm water for around 1 minute to stimulate blood flow and to achieve constant temperature.
Firstly, the middle finger of the left hand (L3) was massaged 3 times from the hand towards the puncture site, then lanced and 5 minutes (+/- 1 minute) later patient was asked to rate the pain intensity during the procedure.
Secondly, the ring finger of the left hand (L4) was massaged 3 times from the hand towards the puncture site, then lanced and 5 minutes later the pain was rated.
Thirdly, the middle finger of the right hand (R3) was massaged 3 times from the hand towards the puncture site, then lanced and again 5 minutes later the pain was rated.
This sequence was followed for all subjects using the lancets as assigned by the randomization list.
Thereafter the completion status was entered in the CRF and the investigator releases the subject from the study. All blood samples taken from subjects were destroyed immediately after measuring of blood volume.
In total 60 subjects were enrolled and each subject had three fingers pricks with a different lancets [5]. Overall, 10 different lancets were investigated, three per subject which leads to 18 capillary blood volume and pain measurements per lancet type.
▪Study population
Male and female subjects with diabetes type I or II were enrolled in the study. Female subjects were allowed to participate in this study only if they were not pregnant in self reporting.
In total 60 diabetic patients were enrolled in the study.
The investigator was obliged to ensure that all patients being considered for the study meet the inclusion and exclusion criteria. The investigator or his/her deputy was also determined to promote compliance to inclusion/exclusion criteria for the duration of the study day. In blindness or poor visibility cases all study instructions were read by a care or accompanying person to the subject (Table 1).
| No | Variable | ||
|---|---|---|---|
| 1 | Number of patients | 60 | 100.0% |
| 2 | Age | 57,4 mean | |
| 3 | Sex Female Male |
31 29 |
51.7% 48.3% |
| 4 | Ethnic origin Caucasian |
60 | 100% |
| 5 | Diabetes Type I Type II |
10 50 |
16.7% 83.3% |
| 6 | Smoking status Smoking Never smoked Past smoker |
11 29 20 |
18.3% 48.3% 33.3% |
| 7 | Patient is Left-handed Right-handed |
2 58 |
3.3% 96.7% |
| 8 | Hand starting the lancing procedure Left Right |
30 30 |
50% 50% |
| 9 | Skin type first lanced finger Thin skin Normal skin Thin skin Very thick skin |
7 37 14 2 |
11.7% 61.7% 23.3% 3.3% |
| 10 | Skin type second lanced finger Thin skin Normal skin Thin skin Very thick skin |
8 36 14 2 |
13.3% 60% 23.3% 3.3% |
| 11 | Skin type third lanced finger Thin skin Normal skin Thin skin Very thick skin |
8 35 15 2 |
13.3% 58.3% 25% 3.3% |
Table 1: Table showing the investigation report.
▪Statistical analysis
All recorded and derived variables are presented using standard procedures (depending on the underlying distribution: for continuous data, sample size, mean, standard deviation (SD), minimum, median and maximum and upper and lower quartile; for categorical data, sample size, absolute and relative frequency).
For sample size 18, a two-sided 95% confidence interval for a single mean extends 0.46 from the observed mean, assuming that the standard deviation is known to be 1 and the confidence interval is based on the t- statistic. In other words, for each lancet the 95% confidence interval extends around 46% of the standard deviation from the observed mean. All data for background and demographic variables were listed by subject. For these parameters summary statistics or frequency tables (race, gender, type of diabetes and skin status) were provided. Efficacy analysis was conducted for the full analysis set (FAS) population principle. All subjects who were lanced with the same lancet and had a blood volume recorded are included in the analysis for that lancet. Efficacy data (blood volume and pain) was listed by subject and lancet.
Descriptive statistics and two-sided 95% confidence intervals based on the t-statistic was calculated for the capillary blood volume per lancet. A similar statistical analysis was performed for the pain perception data.
Results
▪Blood volume
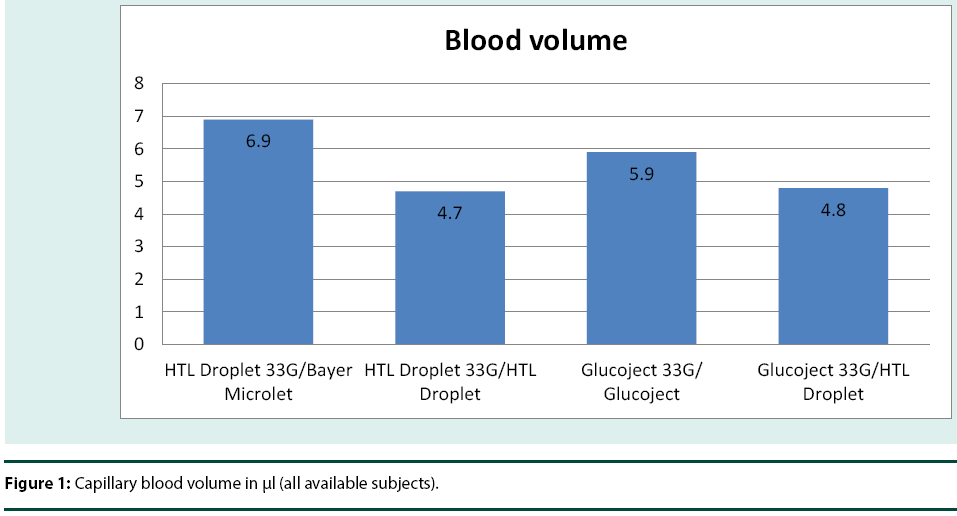
In the study Droplet® personal lancets 33G and Glucoject® personal lancets 33G were used together with Droplet® lancing device, Microlet® lancing device and Glucoject® lancing device. Both had the depth level adjusted to the maximum (Table 2) and (Figure 1).
| No | Lancet Type/Lancing device | N | Mean | STD | Min | Q1 | Median | Q3 | Max | LCL | UCL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HTL Droplet 33G/Bayer Microlet | 18 | 6.9 | 6.2 | 0.3 | 1.9 | 4 | 10.3 | 19 | 3.8 | 9.9 |
| 2 | HTL Droplet 33G/HTL Droplet | 18 | 4.7 | 5.9 | 0 | 0.6 | 2.3 | 7.4 | 22.9 | 1.7 | 7.6 |
| 3 | Glucoject 33G/ Glucoject | 18 | 5.9 | 6.4 | 0.3 | 1.6 | 2.9 | 6.1 | 23.9 | 2.7 | 9 |
| 4 | Glucoject 33G/HTL Droplet | 18 | 4.8 | 3.9 | 0.3 | 1.9 | 3.6 | 7.1 | 14.5 | 2.9 | 6.7 |
Table 2: Summary statistics for Capillary blood volume in μl (all available subjects)
▪Pain Perception
Perceived pain was evaluated as a secondary efficacy variable. The pain perception was measured after each prick. 3 minutes (+/- 1 minute) after pricking the subject noted in his/ her worksheet intensity of the perceived pain (Table 3).
| No | Lancet Type/ Lancing device | N | Min | Q1 | Median | Q3 | Max |
| 1 | HTL Droplet 33G/ Bayer Microlet | 18 | 0 | 1 | 2 | 3 | 3 |
| 2 | HTL Droplet 33G/ HTL Droplet | 18 | 0 | 1 | 2 | 2 | 3 |
| 3 | Glucoject 33G/ Glucoject | 18 | 1 | 1 | 2 | 3 | 5 |
| 4 | Glucoject 33G/ HTL Droplet | 18 | 0 | 1 | 1 | 3 | 8 |
Table 3: Summary statistics for pain rating (0 - no pain, 10 - most imaginable pain).
Conclusion
The average blood volumes obtained with Droplet® personal lancet 33G and Glucoject® personal lancet 33G used in cooperation with Droplet® lancing device, Microlet®2 lancing device and Glucoject® lancing device were in each case higher than 4 μl except for one puncture. In 50% of pricks the obtained average blood volume was higher than 6 μl. Bleeding time was up to 2 minutes. The study results have also shown that in case of majority of patients the received blood sample volume has been higher than 0.5 μl with a total effectiveness rate of 91.67%.
Perceived pain was evaluated as a secondary efficacy variable. The pain perception was measured after each prick [6,7]. 5 minutes (+/- 1 minute) after pricking the subject noted in his/ her worksheet intensity of the perceived pain.
Final Conclusion
As demonstrated by the results of clinical evaluations, Droplet® personal lancets 33G type 560 and Glucoject® personal lancet 33G s type 560 manufactured by HTL-STREFA S.A. provide sufficient blood sample for personal blood glucose level measurements with minimal pain perception.
References
- DCCT Research Group (1993) Diabetes Control and Complications Trial (DCCT): the effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977-986.
- UK Prospective Diabetes Study Group (1995) UK Prospective Diabetes Study 16: overview of 6 years' therapy of type II diabetes: a progressive disease. Diabete s 44: 1249-1258.
- Goldstein DE, Little RR, Lorenz RA, Malone JI, Nathan D, et al. (1995) Peterson CM: Tests of glycemia in diabetes. Diabetes Care 18: 896-909.
- American Diabetes Association (1994) Self-monitoringof blood glucose. Diabetes Care 17: 81-86.
- American Diabetes Association (1985) Self-monitoring of blood glucose. Diabetes Care 8: 515.
- Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, et al. (2002) Defining the relationship between plasma glucose and HbA1c: analysis of glucose profiles and HbA1c in the Diabetes Control and Complications Trial. Diabetes Care 25: 275-278.
- Bär KJ, Brehm S, Boettger MK, Boettger S, Wagner G, et al. (2005) Pain perception in major depression depends on pain modality. Pain 117: 97-103.